Author: BalanceGenics Longevity Research Team (How100.com)
Table of Contents:
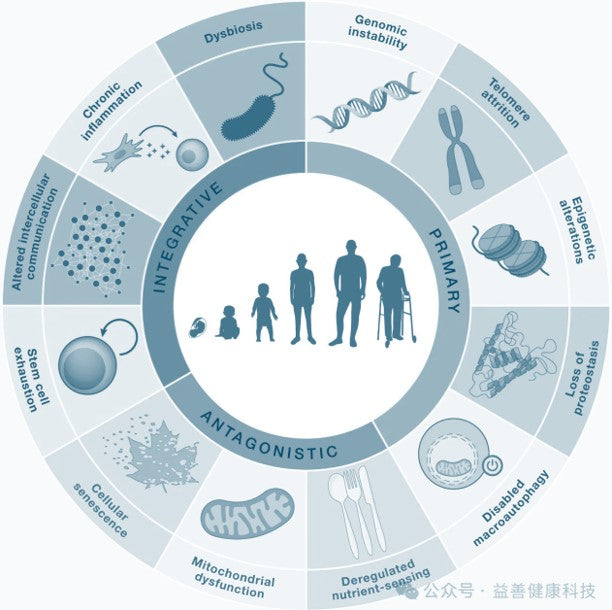
12 Hallmarks of Aging:
- Genomic Instability
- Telomere Attrition
- Epigenetic Alterations
- Loss of Proteostasis
- Disabled Macroautophagy (New):
- Deregulated Nutrient-sensing (DSNS)
- Mitochondrial Dysfunction
- Cellular Senescence
- Stem Cell Exhaustion
- Altered Intercellular Communication
- Chronic Inflammation (New)
- Ecological Dysbiosis (New)
5 Best Supplements to Reverse the 12 Hallmarks of Aging
BalanceGenics 12 Rev-Time
Reference
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased risk of death. This decline is a major risk factor for major human diseases such as cancer, diabetes, cardiovascular disease and neurodegenerative diseases.
A 2013 review published in the Cell journal journal, The Hallmarks of Aging, listed nine common features that represent aging in different organisms, with a special emphasis on mammalian aging. These features/markers such as genomic instability, telomere attrition, et al.
Since the publication of the first edition of the markers of senescence in the journal Cell in 2013, almost 300,000 articles have been published on the subject. As a result, in December 2022, the journal Cell published a review paper titled: Hallmarks of aging: An expanding universe, which updates the hallmarks of aging and contributes to further understanding of the aging process as well as exploring therapeutic strategies for aging interventions.
Hallmarks of aging should fulfill the following three criteria:
(1) they should be age-related manifestations
(2) they can be experimentally aggravated to accelerate aging.
(3) Aging can be slowed down, stopped, or reversed by therapeutic intervention on them.
There are 12 recent common features of aging, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, and macroautophagy. disabled macroautophagy (new), deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion stem cell exhaustion, altered intercellular communication, chronic inflammation (new), and dysbiosis (new).

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
These features interact with each other rather than being independent of each other.
- Genomic Instability

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
The integrity and stability of the genome is threatened by exogenous and endogenous factors. Exogenous factors, such as chemical, physical and biological factors. Endogenous factors such as DNA replication errors, chromosome segregation defects, oxidation, and spontaneous hydrolysis reactions. Genetic damage caused by the above factors is highly diverse and includes point mutations, deletions, translocations, telomere shortening, single and double stranded breaks, chromosomal rearrangements, defects in nuclear structure, and gene disruption caused by viral or transposon integration. Involves damage to nuclear DNA (nDNA) and mitochondrial DNA (mtDNA) as well as chromosome structure and stability. All of these molecular alterations and the resulting genomic chimerism have the potential to contribute to the onset of senescence. Consequently, organisms have evolved a complex network of DNA repair and maintenance mechanisms; however, these DNA repair networks become progressively dysfunctional with age, exacerbating the accumulation of genomic damage and ectopic accumulation of DNA in the cytoplasm. In addition, the genome stability system includes specialized mechanisms for maintaining the proper length and function of telomeres (this is the subject of a separate flag, see below).
- Telomere Attrition

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
Telomere attrition is the gradual shortening of protective caps, known as telomeres, located at chromosome ends. These caps play a vital role in maintaining genomic stability by preventing chromosome degradation or fusion. With each cell division, telomeres naturally shorten due to the end-replication problem. Consequently, telomere length acts as a 'molecular clock,' marking cellular aging. When telomeres become critically short, cells may enter senescence or undergo apoptosis, impairing tissue regeneration. Accelerated by factors like oxidative stress and inflammation, telomere shortening is linked to aging-related diseases.
- Epigenetic Alterations

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
Epigenetic modifications, such as DNA methylation, histone modifications, and non-coding RNA expression, play a crucial role in regulating gene expression and cellular identity. However, with aging, the epigenome undergoes significant alterations, including global changes in DNA methylation patterns and histone modifications. These epigenetic changes can affect gene expression profiles, cellular function, and contribute to age-related phenotypes.
- Loss of Proteostasis
Aging and aging-related diseases, such as amyotrophic lateral sclerosis (ALS), Alzheimer's disease, Parkinson's disease, and cataracts, are associated with impaired protein homeostasis or protease homeostasis. It leads to the accumulation of misfolded, oxidized, glycosylated, or ubiquitinated proteins, which often form aggregates that form intracellular inclusion bodies or extracellular amyloid plaques. Intracellular protein homeostasis may be disrupted due to increased production of mistranslated, misfolded, or incomplete proteins. When mechanisms such as quality control fail, the protein homeostasis network also collapses.

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
- Disabled Macroautophagy (New):
Macroautophagy, commonly referred to as autophagy, is a cellular process responsible for the degradation and recycling of damaged organelles and proteins. It plays a critical role in maintaining cellular homeostasis and defending against stressors such as nutrient deprivation and oxidative stress. However, with aging, autophagic activity declines, leading to the accumulation of dysfunctional organelles and proteins. Disabled macroautophagy contributes to cellular dysfunction and the progression of age-related diseases.
- Deregulated nutrient-sensing (DSNS)

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
The nutrient-sensing network is highly conserved in evolution. This process involves growth hormone (GH), insulin/insulin growth factor-1 (IGF1) and its receptor, the PI3K-AKT and Ras-MEK-ERK pathways, and transcription factors. Rapamycin Complex-1 (MTORC1) Mechanistic targets respond to nutrients, including glucose and amino acids, as well as stressors, such as hypoxia and hypoenergetics, to regulate the activity of many proteins, including transcription factors, such as SREBP and TFEB. Nutrient-sensing networks are central regulators of cellular activity, including autophagy, mRNA and ribosome biogenesis, and protein synthesis, glucose, nucleotide and lipid metabolism, mitochondrial biogenesis and proteasome activity.
- Mitochondrial Dysfunction

With age, mitochondrial dysfunction occurs due to multiple intertwined mechanisms (including accumulation of mtDNA mutations, defects in protease homeostasis leading to destabilization of the respiratory chain complex, reduced organelle turnover, and changes in mitochondrial dynamics). This in turn enhances ROS production and may trigger high permeability of the mitochondrial membrane, leading to inflammation and cell death.
- Cellular Senescence

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
Cellular senescence is a response caused by acute or chronic injury. In humans, senescent cells accumulate at different rates in a variety of inflammatory conditions, although all cell types undergo senescence during the aging process, a process triggered, at least in part, by telomeres that shorten with aging. The most compelling evidence for the causal role of cellular senescence in the aging process is that sustained elimination of senescent cells by genetic or pharmacologic routes prolongs the health and lifespan of naturally aging mice.
- Stem Cell Exhaustion

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
Aging is associated with reduced tissue renewal in the steady state and impaired tissue repair after injury. Each organ has its own renewal and repair strategy. Tissue repair requires a modified microenvironment that promotes de-differentiation and plasticity of cells at different tissue sites through the secretion of cytokines (partly due to senescence-associated secretory responses), growth factors, and extracellular matrix (ECM) modulators. These injury-induced plastic cells may acquire pluripotent progenitor cell functions, i.e., "cellular reprogramming". Stem cell depletion is due to the loss of cellular plasticity required for tissue repair. Therefore, stem cell depletion is one of the hallmarks of aging.
- Altered Intercellular Communication
Aging is associated with progressive alterations in intercellular communication, impairing homeostasis and hormonal regulation. Aging involves defects in neural, neuroendocrine and hormonal signaling pathways. Existing studies have explored pro-senescence bloodborne factors, anti-aging bloodborne factors, long- and short-range communication systems, and extracellular matrix (ECM) aspects.
- Chronic Inflammation (new)

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
Chronic inflammation, a hallmark of aging, is characterized by a sustained and dysregulated immune response that persists over time, contributing to various age-related diseases. Factors such as cellular senescence, mitochondrial dysfunction, and dysregulated immune signaling pathways drive this inflammatory state. Senescent cells release inflammatory molecules, while mitochondrial dysfunction generates reactive oxygen species (ROS), both triggering inflammation. Dysregulation of immune signaling pathways like NF-κB further exacerbates inflammation. Chronic inflammation promotes tissue damage, impairs cellular function, and disrupts homeostasis, fueling the progression of conditions like cardiovascular disease, neurodegenerative disorders, and cancer. Lifestyle interventions such as diet and exercise can help mitigate inflammation, while targeted therapies aiming to modulate the immune response offer potential for improving health outcomes in aging populations.
- Ecological Dysbiosis (new)

Source: López-Otín,C., et al. (2022): Hallmarks of aging: An expanding universe
The intestinal flora plays a vital role in various physiological processes, including digestion, nutrient absorption, and protection against pathogens. As we age, changes occur in the structure and activity of gut flora communities, resulting in reduced diversity and beneficial flora levels while harmful flora increases. This dysregulation contributes to aging-related conditions like obesity, diabetes, cancer, cardiovascular disease, neurological disorders, and inflammation. Studies, including metabolomics and fecal microbiota transplantation (FMT), highlight the causal link between aging and ecological dysregulation. Interventions aimed at restoring the gut ecology may extend healthy lifespan.
In Summary
There are strong correlations between the 12 aging hallmarks. For example, genomic instability (including instability caused by telomere shortening) cross-impacts epigenetic alterations (e.g., through inactivating mutations in epigenetic modifiers such as TET2), loss of protein homeostasis (e.g., due to the production of mutated, misfolded proteins), incapacitating macroautophagy (e.g., the ability to remove redundant centrosomes, extra-nuclear chromatin, and cytoplasmic DNA through autophagy), nutritional sensing dysregulation (e.g., because SIRT6 is a NAD + sensor involved in DNA repair but also responds to nutrient deficiencies), mitochondrial dysfunction (e.g., due to mtDNA mutations), cellular senescence (e.g., due to DNA damage triggering senescence), altered inter-cellular communication (e.g., by blocking activation of communication pathways), chronic inflammation (e.g., due to CHIP and DNA leakage into the cytoplasm leading to inflammation) and ecological dysregulation (e.g., because mutations in intestinal cells favor ecological dysregulation and specific microbial proteins and metabolites act as mutagens). Most, if not all, of the features of aging can be listed with similar functional relationships, illustrating their strong interconnections. These features interact with each other rather than being independent of each other.
5 Best Supplements to Reverse the 12 Hallmarks of Aging
Here is a list of several supplements renowned for their potential in reversing various hallmarks of aging:
- SPD Spermidine
- Reverses all 12 hallmarks of aging, including genomic instability, telomere attrition, and chronic inflammation.
- Stimulates autophagy and acts as a calorie regulation mimetic.
- Protects against neurodegenerative disorders like Alzheimer’s and enhances fertility.
- NMN (Nicotinamide Mononucleotide)
- Targets 9 out of 12 hallmarks of aging, such as genomic instability, cellular senescence, and chronic inflammation.
- Improves mitochondrial bioenergetics and NAD+ levels.
- Enhances stem cell regeneration and immune system function.
- CAaKG Calcium Alpha-Ketoglutarate
- Addresses 4 hallmarks of aging, including epigenetic alterations and chronic inflammation.
- Improves insulin sensitivity and glucose homeostasis.
- Reduces inflammation markers and cellular response to aging-associated inflammation.
- Matcha
- Reduces 4 out of 12 hallmarks of aging, including cellular senescence and loss of proteostasis.
- Targets genes implicated in inflammation, cancer, and neurodegenerative diseases.
- Prevents liver damage and promotes telomere length maintenance.
- Fisetin & Quercetin
- Fisetin addresses all 6 hallmarks of aging, including genomic instability and mitochondrial dysfunction.
- Quercetin targets genomic instability, cellular senescence, and chronic inflammation.
- Both compounds extend lifespan and protect against age-related illnesses.
These supplements offer a comprehensive approach to combating aging by targeting multiple hallmarks simultaneously. Incorporating them into one's daily routine may provide significant benefits in promoting longevity and overall health.
BalanceGenics 12 Rev-Time:
BalanceGenics 12 Rev-Time is a high-quality supplement designed to reverse the 12 hallmarks of aging. Our scientifically-backed formula aims to help you achieve your health goals while slowing your aging process.
Key Ingredients and Benefits:
- Calcium Alpha-Ketoglutarate (1000mg /daily): Provides a key metabolite required for cellular energy production, enhancing exercise performance.
-
Quercetin (500mg /daily):
Supports the immune system and helps clear out senescent "zombie" cells.
-
Pterostilbene (250mg /daily):
Activates sirtuin proteins that regulate cellular and metabolic processes, maintaining the epigenome.
-
Fisetin (100mg /daily):
A senolytic compound that removes senescent cells and supports immunity.
-
Spermidine (900mcg /daily):
A potent inducer of autophagy, enhancing mitochondrial function.
For more information, please see BalanceGenics Product page:
BalanceGenics 12 Rev-Time (Reverse 12 Hallmarks of Aging)
BalanceGenics: Personalized One-Stop Longevity Platform (How100.com)
Launched in California in 2018, BalanceGenics ("How100") started out as a solution to our own needs. Our team consists of seasoned health experts, doctors, and entrepreneurs with a common interest in anti-aging.
We use the latest findings from global leading longevity scientists to develop products and services, focusing on Longevity Supplements (cellular anti-aging) and Physical Therapy Exercises (physical anti-aging).
BalanceGenics' Mission is to create personalized solutions to live longer but stay younger.
We will be your personalized one-stop anti-aging platform and help you stay younger for longer.
References:
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194-1217. https://doi.org/10.1016/j.cell.2013.05.039
López-Otín C, Blasco M. A., Partridge L, Serrano M., &, Kroemer G.(2022) Hallmarks of aging: An expanding universe. Cell, S0092-8674(22)01377-0. doi: 10.1016/j.cell.2022.11.001. Epub ahead of print. PMID: 36599349.
Gems, D., & de Magalhães, J. P. (2021). The hoverfly and the wasp: A critique of the hallmarks of aging as a paradigm. Ageing Research Reviews, 70, 101407. https://doi.org/10.1016/j.arr.2021.101407
Fraser, H. C., Kuan, V., Johnen, R., Zwierzyna, M., Hingorani, A. D., Beyer, A., & Partridge, L. (2022). Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell, 21(3), e13524. https://doi.org/10.1111/acel.13524
Pekar, Thomas, et al. “The Positive Effect of Spermidine in Older Adults Suffering from Dementia.” Wiener Klinische Wochenschrift, vol. 133, no. 9, 2021, pp. 484–491, www.ncbi.nlm.nih.gov/pmc/articles/PMC8116233/, https://doi.org/10.1007/s00508-020-01758-y.
Keshavarz, Maryam, et al. “Targeting the “Hallmarks of Aging” to Slow Aging and Treat Age-Related Disease: Fact or Fiction?” Molecular Psychiatry, 15 July 2022, https://doi.org/10.1038/s41380-022-01680-x.
Gyanwali, Bibek, et al. “Alpha-Ketoglutarate Dietary Supplementation to Improve Health in Humans.” Trends in Endocrinology & Metabolism, vol. 33, no. 2, Feb. 2022, pp. 136–146, https://doi.org/10.1016/j.tem.2021.11.003.
van der Rijt, Sanne, et al. “Integrating the Hallmarks of Aging throughout the Tree of Life: A Focus on Mitochondrial Dysfunction.” Frontiers in Cell and Developmental Biology, vol. 8, 26 Nov. 2020, https://doi.org/10.3389/fcell.2020.594416.
Elsallabi, Osama, et al. “Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection.” Molecules, vol. 27, no. 3, 23 Jan. 2022, p. 738, , https://doi.org/10.3390/molecules27030738.
Khan, Naghma, et al. “Fisetin: A Dietary Antioxidant for Health Promotion.” Antioxidants & Redox Signaling, vol. 19, no. 2, 10 July 2013, pp. 151–162, https://doi.org/10.1089/ars.2012.4901.
Harish Chandra Pal, et al. “Fisetin Inhibits UVB‐Induced Cutaneous Inflammation and Activation of PI3K/AKT/NFκB Signaling Pathways in SKH‐1 Hairless Mice.” Photochemistry and Photobiology, vol. 91, no. 1, 7 Oct. 2014, pp. 225–234,, https://doi.org/10.1111/php.12337.
Robbins, Paul D., et al. “Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span.” Annual Review of Pharmacology and Toxicology, vol. 61, no. 1, 6 Jan. 2021, pp. 779–803, https://doi.org/10.1146/annurev-pharmtox-050120-105018.