Author: BalanceGenics Anti-aging Research Team (How100.com)
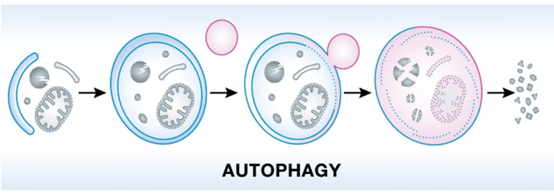
Autophagy, a cellular process that degrades and recycles damaged organelles and proteins, plays a crucial role in maintaining cellular homeostasis and preventing age-related diseases. However, the efficiency of the autophagic machinery declines with age, leading to the accumulation of cellular waste and impairment of cellular function.

Source: https://phys.org/
Autophagy‘s key roles in the aging process and longevity
- Cellular Rejuvenation:
Autophagy helps to remove damaged or dysfunctional organelles, misfolded proteins, and other cellular waste, which can accumulate during aging.By clearing these potentially harmful components, autophagy contributes to the maintenance of cellular health and function, slowing down the aging process.
- Metabolic Regulation:
Autophagy can regulate cellular metabolism, energy homeostasis, and the availability of nutrients, all of which are important for healthy aging.Proper metabolic regulation through autophagy can help prevent age-related diseases, such as metabolic disorders and neurodegeneration.
- Stem Cell Maintenance:
Autophagy plays a crucial role in the maintenance and function of stem cells, which are essential for tissue regeneration and repair.By preserving the health and stemness of stem cells, autophagy can contribute to longevity and the delay of age-related tissue degeneration.
- Immune Function:
Autophagy is involved in the regulation of the immune system, which is essential for fighting off infections and diseases that can contribute to aging.
Impaired autophagy has been linked to age-related immune dysfunction, which can lead to increased susceptibility to infections and chronic inflammation.
- Neuroprotection:
Autophagy is important for the clearance of misfolded proteins and damaged organelles in neurons, which can accumulate during aging and contribute to neurodegenerative diseases.
Maintaining proper autophagy in the brain can help prevent age-related cognitive decline and neurodegeneration, thereby promoting longevity.
Research findings about interplay between Autophagy and Aging
Several studies have highlighted the complex interplay between autophagy and aging. One of the hallmarks of aging is the loss of proteostasis, characterized by the accumulation of irreparably damaged proteins that distract chaperones from folding healthy proteins, driving cellular aging and death. Malfunctioning of autophagy with age may result in systemic diseases such as diabetes, vascular disease, and organ-specific pathologies like sarcopenia and neurodegenerative diseases. Hallmarks of aging, including an increase in ROS, loss of proteostasis, genome instability, and telomere exhaustion, are closely linked to the dysregulation of autophagy.
Numerous animal studies have provided evidence that promoting autophagy can extend lifespan. For example, Shen et al. (2018) found that overexpression of the autophagy gene Atg5 in mice increased median lifespan by 17% in males and 11% in females. Similarly, administration of rapamycin, a pharmacological inducer of autophagy, has been shown to extend lifespan in various model organisms, including mice. Conversely, genetic manipulations that inhibit autophagy, such as knockdown of Atg7, have been found to shorten lifespan in worms and flies .
The age-related decline in autophagic activity has been linked to the development of various age-related diseases. In the cardiovascular system, impaired autophagy accelerates cardiac and vascular aging, leading to endothelial dysfunction, atherosclerosis, and heart failure. Boyle et al. (2020) demonstrated that restoring autophagy through exercise training or caloric restriction can improve cardiac function and delay the onset of age-related heart disease in mice.
In the brain, dysfunctional autophagy is implicated in the pathogenesis of neurodegenerative disorders, such as Alzheimer's and Parkinson's disease. Komatsu et al. (2006) found that deletion of the autophagy gene Atg7 in the mouse brain led to the accumulation of ubiquitinated protein aggregates and neurodegeneration. Conversely, enhancing autophagy through pharmacological interventions or genetic manipulations has been shown to reduce the accumulation of toxic protein aggregates and improve neuronal function in animal models of neurodegenerative diseases.

Source: www.nature.com
In cancer, autophagy plays a dual role, acting as a tumor suppressor in the early stages of tumorigenesis by preventing genomic instability and the accumulation of damaged organelles, while promoting tumor growth and survival in established cancers by providing nutrients and energy during periods of stress. Inhibition of autophagy has been explored as a potential therapeutic strategy in cancer, with several autophagy inhibitors currently in clinical trial.
In conclusion: the research results highlight the critical role of autophagy in maintaining cellular homeostasis and preventing age-related diseases.
The age-related decline in autophagic activity contributes to the development of various hallmarks of aging and is implicated in the pathogenesis of numerous age-related diseases. Modulating autophagy through genetic, pharmacological, or lifestyle interventions has shown promise in extending lifespan and improving health outcomes in animal models. However, further research is needed to fully understand the complex interplay between autophagy and aging and to develop targeted interventions that can effectively enhance autophagic function and promote healthy aging in humans.
BalanceGenics: Personalized One-Stop Anti-aging Platform (How100.com)
Launched in California in 2018, BalanceGenics ("How100") started out as a solution to our own needs. Our team consists of seasoned health experts, doctors, and entrepreneurs with a common interest in anti-aging.
We use the latest findings from global leading longevity scientists to develop products and services.
BalanceGenics' Mission is to create personalized solutions to live longer but stay younger.
We will be your personalized one-stop anti-aging platform and help you stay younger for longer.
Reference:
Proteostasis collapse is a driver of cell aging and death - PNAS. www.pnas.org › full › pnas.1906592116
Hallmarks of Aging: An Autophagic Perspective - PMC - NCBI. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6333684/
Rubinsztein, D. C., Mariño, G., & Kroemer, G. (2011). Autophagy and aging. Cell, 146(5), 682-695. [https://doi.org/10.1016/j.cell.2011.07.030]
Madeo, F., Zimmermann, A., Maiuri, M. C., & Kroemer, G. (2015). Essential role for autophagy in life span extension. The Journal of clinical investigation, 125(1), 85-93. [https://doi.org/10.1172/JCI73946]
García-Prat, L., Martínez-Vicente, M., Perdiguero, E., Ortet, L., Rodríguez-Ubreva, J., Rebollo, E., ... & Muñoz-Cánoves, P. (2016). Autophagy maintains stemness by preventing senescence. Nature, 529(7584), 37-42. [https://doi.org/10.1038/nature16187]
Deretic, V., Saitoh, T., & Akira, S. (2013). Autophagy links innate and adaptive immunity. Nature immunology, 14(5), 460-467. [https://doi.org/10.1038/ni.2557]
Menzies, F. M., Fleming, A., & Rubinsztein, D. C. (2015). Compromised autophagy and neurodegenerative diseases. Nature reviews neuroscience, 16(6), 345-357. [https://doi.org/10.1038/nrn3961]
Shen, C., Tian, G., Liang, J., Weng, X., & Li, S. (2018). Overexpression of autophagy-related gene Atg5 extends life span in transgenic mice. Aging Cell, 17(6), e12834.
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., ... & Miller, R. A. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460(7253), 392-395.
Tóth, M. L., Sigmond, T., Borsos, É., Barna, J., Erdélyi, P., Takács-Vellai, K., ... & Vellai, T. (2008). Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy, 4(3), 330-338.
Autophagy in Cardiovascular Aging Circulation Research - AHA Journals. https://www.ahajournals.org/doi/full/10.1161/CIRCRESAHA.118.312208